what starting compound(s) would you use in an aldol reaction to prepare as the major product:
Aldehydes and Ketones
1. Which of the following is a correct proper noun for (CH3)2C=CHCOCH3?
A) 2-methyl-2-penten-4-ane
B) 4-methyl-3-penten-ii-one
C) 1,three-dimethyl-2-pentenal
D) isopentenone
ii. Which of the following is 3,iii-diphenylpropanal?
A) Chalf-dozenHvCH2CH(Chalf-dozenHfive)CHO
B) Chalf-dozenH5CH2CH2COC6H5
C) (Chalf-dozenH5)iiCHCHiiCHO
D) (Chalf-dozenH5)iiCHCH2COChalf dozenH5
3. Which of the following compounds is not named correctly?
| A) ii-methyl-3-heptanone (CH3)2CHCOCHtwoCH2CH2CH3 |
| B) phenylacetaldehyde Chalf-dozenHfiveCHiiCHO |
| C) 4-hexyn-ii-i CH3COCH2C≡CCH3 |
| D) para-bromoacetophenone p-BrC6H4CHtwoCOCHiii |
iv. Which of the following compounds is not named correctly?
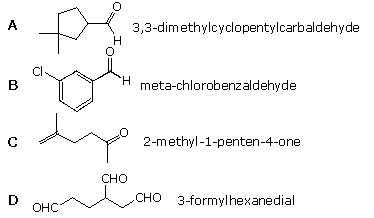 | |
five. Which of the following compounds is not named correctly?
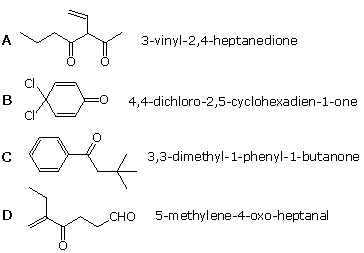 | |
| | |
| | |
6 Which of the following may be classified every bit an acetal?
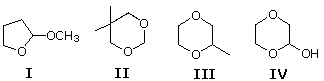
A) I & II
B) III & IV
C) only IV
D) all but IV
seven Which of the following reactions is a skilful method for preparing an aldehyde?
A) Jones' reagent and a 1º-alcohol
B) Jones' reagent and a 2º-alcohol
C) PCC and a 1º-alcohol
D) HtwoSO4 a 1º-booze and heat
8 Which of the post-obit are enol tautomers of 3-methylcyclohexanone?

A) I & II
B) I & Iv
C) 2 & III
D) only I
9 Which of the following statements is not by and large true?
A) C=O is stronger than an equivalent C=C
B) C=O has a larger bond dipole than C=C
C) aldehydes and ketones have higher boiling points than similarly sized alkenes
D) alkenes add nucleophiles more rapidly than aldehydes or ketones of similar construction
x Which of the following compounds exchanges the largest number of hydrogens for deuterium when treated with KOD in D2O?
A) 3-methyl-ane,two-cycloheptanedione
B) 2-methyl-1,3-cycloheptanedione
C) 5-methyl-i,3-cycloheptanedione
D) 6-methyl-1,4-cycloheptanedione
11 Four C8HxivO ketones are examined by 13Cnmr spectroscopy.
One of them has 5 distinct carbon signals. Which of the post-obit fits this fact?
A) 4,iv-dimethylcyclohexanone
B) 3,iii-dimethylcyclohexanone
C) ii,2-dimethylcyclohexanone
D) two,2,4,4-tetramethylcyclobutanone
12 Which of the following compounds could not exist converted to pentanal in ane or ii steps?
A) 1-pentyne
B) trans-v-decene
C) 2-pentanone
D) 1-pentanol
13 Treatment of cyclohexanone with an backlog of H2 18O produces 18O labeled cyclohexanone.
Which of the following is a likely intermediate in this isotope exchange? (the isotope is not named)
A) 1-cyclohexen-1-ol
B) i,1-cyclohexanediol
C) 2-cyclohexen-one-one
D) 1,2-cyclohexanediol
14 Reaction of CviHfiveCHBr2 with NaOH in aqueous THF is likely to produce which product?
A) C6H5CHBrOH
B) C6H5CH(OH)two
C) Chalf-dozenH5CHO
D) Chalf-dozenH5CO2H
15 Which of the following carbonyl compounds reacts most rapidly with nucleophilic reagents?
A) benzaldehyde
B) 3,3-dimethylbutanal
C) acetophenone
D) two,2-dimethylcyclohexanone
16 Which of the following amines would be best called for preparing an enamine derivative from cyclohexanone?
A) dimethylamine
B) ethylamine
C) trimethylamine
D) hydroxylamine
17 Which reaction or sequence of reactions would best accomplish the following synthesis?
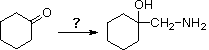
A) CHiiiNHtwo, acrid catalyst & rut
B) CH2=NH, acid catalyst & oestrus
C) (i) NH3 acid goad & heat; (ii) CH2I2 & Zn(Cu)
D) (i) HCN & NaCN; (ii) LiAlHiv in ether
18 Heating cyclopentanone with either: I ethyl amine, or Two diethylamine, together with an acid catalyst leads to different results.
Which of the post-obit best describes this divergence?
A) I gives an imine & II fails to react
B) I gives an enamine & Two fails to react
C) I gives an imine & Two gives an enamine
D) I gives an enamine & II gives an imine
19 Which reaction or sequence of reactions would be best used to convert cyclohexanone to cis-1,2-cyclohexanediol?
A) PCC in CH2Clii and base
B) (i) NaBH4; (two) H3PO4 & heat; (3) OsO4 in pyridine
C) (i) NaBHfour; (two) H3PO4 & heat; (iii) Chalf dozenH5CO3H
D) (i) NaBH4; (2) OsO4 in pyridine
20 A C9H10O chemical compound has a strong absorption at 1686 -1cm in the infrared.
Which of the following compounds would be expected to display such an absorption band?
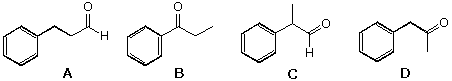 | |||
21 A C9H10O chemical compound has a strong absorption at 1730 and ii smaller simply sharp absorption peaks at 2719 & 2818 -onecm in the infrared. The iHnmr has a strong doublet at δ1.0 ppm.
Which of the following compounds would be expected to brandish these features?
 | |||
22 The Wittig reaction takes place between a carbonyl compound and a phosphorus ylid.
Which of the following represents the dipolar correspondent to such an ylide?
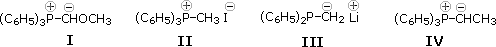
A) I & 2
B) II only
C) 3 merely
D) I & Iv
23 Which of the post-obit reaction sequences would exist best for converting cyclohexanol to methylenecyclohexane, (CH2)fiveC=CH2 ?
A) (i) H3PO4 & heat; (2) CH2I2 + Zn(Cu)
B) (i) PCC in CH2Cltwo; (ii) CHiiiMgBr; (3) H3PO4 & rut
C) PCC in CH2Cltwo; (ii) (C6Hfive)3P=CH2
D) CH2N2 & heat
24 Which of the following Wittig reagents would exist useful for converting R2C=O into R2CHCH=O?
A) (Chalf-dozenH5)3P=CH-OCH3
B) (Chalf dozenHfive)threeP=CH-CHthree
C) (C6H5)3P=Cl2
D) (C6H5)3P=CH-CH=CH2
25 Ii equivalents of the Wittig reagent (CHiii)twoC=CH-CH=P(C6H5)3 were allowed to react with a CivHivO2 chemical compound.
The chief production was 2,11-dimethyl-2,iv,6,8,10-dodecapentaene, (CHthree)2C=CH(CH=CH)3CH=C(CHthree)ii.
What was the CfourHfourO2 compound used in this reaction?
A) ii-butyne-i,4-diol
B) i,ii-cyclobutanedione
C) 1,three-butadien-2,3-diol
D) 2-butenedial
26 Which of the post-obit reactions would not be a useful manner of preparing 1-phenyl-2-butanol?
A) phenylacetaldehyde + ethylmagnesium bromide
B) butanal + phenylmagnesium bromine
C) propanal + benzylmagnesium bromine
D) i-phenyl-2-butanone + NaBHiv
27 Which of the following reactions would not be a useful way of preparing 2-phenyl-2-butanol?
A) 2-butanone + phenylmagnesium bromine
B) acetophenone + ethylmagnesium bromide
C) cis-2,3-dimethyloxirane + phenylmagnesium bromide
D) ethylphenylketone + methylmagnesium iodide
28 In the reaction of (R)-3-phenyl-2-butanone with methylmagnesium iodide, what happens to the configuration of the stereogenic center?
A) nothing, it remains unchanged
B) inversion takes place
C) racemization occurs
D) the product is achiral
29 Which of the post-obit reactions would not be useful for converting four,iv-diethylcyclohexanone into ane,1-diethylcyclohexane?
A) Wolff-Kishner reduction (N2H4, strong base & heat)
B) Clemmensen reduction (Zn/Hg, acid & rut)
C) thioacetal reduction (i HSCH2CH2SH & BFthree; 2 Hii + Raney Ni)
D) LiAlHfour in THF & rut
30 Which of the following is a semicarbazone derivative of an aldehyde (RCHO)?
A) RCH=N-NHCONH2
B) RCH=N-OH
C) RCH=Northward-NHtwo
D) RCH=N-C(CH3)three
31 Which of the following isomers is most acidic ?
A) three,4-hexanedione
B) 2,5-hexanedione
C) 2,iv-hexanedione
D) hexanedial
32 You lot have 2 C6H10O ketones, I and II. Both are optically active, only I is racemized by handling with base and II is not.
Wolff-Kishner reduction of both ketones gives the same achiral hydrocarbon, formula CviH12.
What reasonable structures may be assigned to I and II?
A) I is 3-methyl-4-penten-2-one; II is four-methyl-ane-penten-3-one
B) I is 2-methylcyclopentanone; Ii is 3-methylcyclopentanone
C) I is 3-methylcyclopentanone; II is ii-methylcyclopentanone
D) I is ii-ethylcyclobutanone; 2 is 3-ethylcyclobutanone
33 Jones' reagent oxidizes aldehydes to carboxylic acids just normally does not oxidize ketones.
What intermediate species is most likely responsible for the aldehyde oxidation?
A) a carbonyl hydrate
B) an enol tautomer
C) an oxonium conjugate acid of the aldehyde
D) an acylium cation
34 The lithium enolate base from cyclohexanone reacts with alkyl halides, often in dissimilar ways.
Every bit shown here, methyl iodide and tert-butyl bromide react to give different organic products, I and 2, together with lithium halides.
What are the products from these reactions?
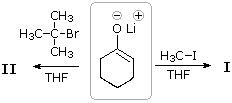
A) I is ii-methylcyclohexanone; 2 is 2-t-butylcyclohexanone
B) I is 1-methoxycyclohexene; II is 1-t-butoxycyclohexene
C) I is 2-methylcyclohexanone; II is 1-t-butoxycyclohexene
D) I is two-methylcyclohexanone; Two is a mixture of cyclohexanone and two-methylpropene
35 A C5H12O compound is optically active, and is oxidized by PCC in CH2Cl2 to an optically agile C5H1OO production, which is racemized in acid or base.
Which of the post-obit best fits these facts?
A) ii-pentanol
B) 2-methoxybutane
C) 2-methyl-1-butanol
D) three-methyl-1-butanol
36 Which of the following aldehydes, used solitary, will undergo an aldol reaction?
A) formaldehyde, CHiiO
B) butanal, CH3(CHii)iiCHO
C) benzaldehyde, C6H5CHO
D) 2-propenal, CHtwo=CHCHO
37 Which of the following reaction sequences would exist best for preparing two,2-dimethyl-iii-hexanone from butanal?
A) (i) addition of tert-butylmagnesium bromide in ether; (ii) hydrolysis workup; (three) PCC in CHtwoCl2
B) (i) NaBH4; (ii) PBrthree; (iii) Mg in ether; (iv) 2,2-dimethylpropanal, followed by hydrolysis
C) (i) Wittig reaction with (CsixH5)3P=C(CH3)2; (ii) BH3 in THF; (iii) H2O2 & base; (iv) PCC in CH2Cl2
D) (i) Wittig reaction with (C6H5)iiiP=CHC(CH3)3; (ii) BH3 in THF; (iii) HiiO2 & base; (iv) PCC in CH2Cltwo
38 How tin can one,i,1,2,ii-pentadeutero-3,3-dimethylpentane, C2HvC(CH3)2C2D5, be prepared from 3,3-dimethyl-2-pentanone?
A) (i) NaOD in D2O (excess); (ii) LiAlD4 in ether; (3) DtwoO workup
B) (i) Wolff-Kishner reduction with N2D4 in ROD; (2) NaOD in D2O (backlog)
C) (i) Zn & DCl in CH3COiiD; (ii) LiAlD4 in ether; (three) D2O workup
D) (i) NaOD in DiiO (backlog); (ii) HSCH2CH2SH + BF3; (iii) D2 + Raney Ni catalyst
39 Acetaldehyde reacts with (R)-one,two-propanediol (acid catalyst) to requite 2 isomeric acetals. What is their isomeric relationship?
A) they are constitutional isomers
B) 2 they are diastereomers
C) three they are enantiomers
D) they are conformers
40 What is the production of the post-obit reaction?
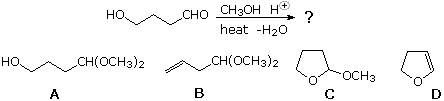 | ||||
41 What product or products are expected from acid-catalyzed hydrolysis of the following compound?
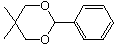
A) just the starting material itself (no reaction)
B) benzaldehyde and 2,two-dimethyl-1,iii-propanediol
C) benzoic acrid and 2,2-dimethyl-one,iii-propanediol
D) 2-phenyl-one,iii-propanediol and acetone
42 Base induced emptying of 3-chlorocyclohexanone I is much faster than elimination of cyclohexyl chloride II.
What is the major cistron bookkeeping for this difference?
A) the halogen in I is less hindered
B) the halogen in I is forced to be axial
C) emptying of I produces a more stable conjugated double bond
D) I chop-chop forms an enolate anion, and this immediately eliminates a stable chloride ion
43 Which of the post-obit optically active compounds volition be racemized on treatment with warm alcoholic sodium ethoxide?
| (R)-2,ii,6-trimethylcyclohexanone | (R)-ii,two,v-trimethylcyclohexanone | (R)-ii-methyl-two-phenylcyclohexanone | (R)-4-methyl-2-cyclohexen-1-one |
| I | II | Iii | IV |
|---|
B) I and III
C) I and IV
D) all compounds volition be racemized
44 A C5H8 hydrocarbon is reacted with BH3 in THF, followed by oxidation with alkali metal hydrogen peroxide.
Treatment of the resulting product with PCC in CH2Cl2 produces a chiral ketone, formula CvH8O.
What hydrocarbon best fits these facts?
A) i-methylcyclobutene
B) methylenecyclobutane
C) vinylcyclopropane
D) cyclopentene
45 Which of the following compounds would not be a possible product from the mixed aldol reaction of acetaldehyde and butanal?
A) 3-hydroxybutanal
B) two-ethyl-iii-hydroxybutanal
C) 3-ethyl-2-hydroxyhexanal
D) 3-hydroxyhexanal
46 A C7H12O2 compound gives a positive Tollens' silver mirror test and a positive iodoform examination.
Which of the following would satisfy these facts?
A) 2-hydroxy-3,3-dimethylcyclopentanone
B) ii,5-heptanedione
C) 2,ii-dimethyl-three-oxopentanal
D) 2,2-dimethyl-4-oxopentanal
47 The iodoform exam for methyl ketones is not widely used anymore.
Which of the post-obit spectroscopic tools is all-time for providing equivalent information?
A) UV-Visible
B) iH nmr
C) 13C nmr
D) infrared
48 An aldol condensation is used to prepare 1,3-diphenyl-two-propenone, CviH5CH=CHCOC6H5.
Which combination of reactants will lead to this production?
A) enolate donor=acetaldehyde; carbonyl acceptor=benzaldehyde
B) enolate donor=phenylacetaldehyde; carbonyl acceptor=phenylacetaldehyde
C) enolate donor=acetophenone; carbonyl acceptor=benzaldehyde
D) enolate donor=propiophenone; carbonyl acceptor=benzaldehyde
49 Which of the following compounds would be the major production from aldol condensation of 6-oxoheptanal?
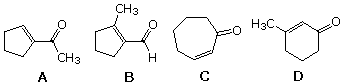 | |||
50 Which of the following procedures would not exist suitable for preparing 3-methyl-1-phenyl-1-butanone, C6H5COCH2CH(CH3)ii?
A) (i) C6H5COCH=CHCH3 + (CH3)2CuLi in ether; (two) H3O+ workup
B) (i) benzene + (CH3)twoCHCH2COCl & AlCl3 (2) H3O+ workup
C) (i) Chalf dozenHvMgBr + (CH3)2CHCH2CHO in ether; (ii) H3O+ workup; (iii) PCC in CH2Cl2
D) (i) (CH3)2CHMgBr + CsixH5COCH3 in ether; (ii) H3O+ workup; (iii) PCC in CH2Cl2
51 Formulas for iv ethyl ethers are drawn beneath. Two are broken by aqueous acid much more hands than the other ii.
Which ethers are more easily hydrolyzed?
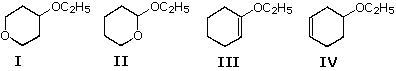
A) I and II
B) Ii and III
C) III and 4
D) I and 4
52 The Wieland-Miescher ketone is an important synthetic intermediate. Its formula is shown beneath.
What starting materials would exist suitable for preparing this compound by a combination of Michael and aldol reactions?
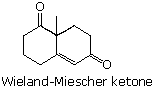
A) 4-methyl-2-cyclohexen-1-i and three-butenal
B) 2-methylcyclohexane-ane,3-dione and three-buten-2-one
C) 2-methyl-2-vinyl-three-cyclohexen-1-1 and acetaldehyde
D) 2-methyl-2-cyclohexen-ane-ane and 1,4-dichlorobutan-two-ane
53 2,ii-Dimethyl-i,three-propanediol is coveniently prepared past heating ii-methylpropanal with backlog formaldehyde and Ca(OH)2.
What sequence of reactions takes place in this synthesis?
A) dehydrogenation to two-methyl-2-propenal followed by addition of formaldehyde
B) dehydrogenation to dimethylketene followed by addition of formaldehyde
C) a crossed aldol reaction followed past a crossed Cannizzaro reaction
D) a crossed Cannizzaro reaction followed by a crossed aldol reaction
54 A bis-aldol dimerization of 1-phenyl-1,2-propanedione, CviH5COCOCH3, gives which of the following?
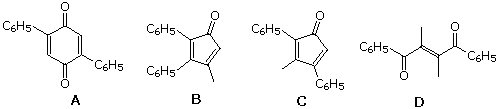 | ||||
55 What donor and acceptor reactants should be used to set the following chemical compound past an aldol reaction?
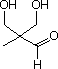
A) acceptor = formaldehyde; donor = propanedial
B) acceptor = 1,iii-propanediol; donor = ethanal
C) acceptor = propanal; donor = formaldehyde
D) acceptor = formaldehyde; donor = propanal
56 What donor and acceptor reactants should be used to prepare the following chemical compound by an aldol condensation (or Claisen-Schmidt reaction)?
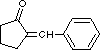
A) acceptor = cyclopentanone; donor = benzaldehyde
B) acceptor = benzaldehyde; donor = cyclopentanone
C) acceptor = phenylacetaldehyde; donor = cyclopentanone
D) acceptor = cyclopentanecarbaldehyde; donor = toluene
57 The formula of brevicomin, a pheremone of the western pine protrude, is shown below.
What open concatenation ketodiol would close to this bicyclic acetal? (ignore stereoisomer problems)
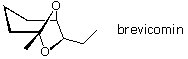
A) seven,viii-dihydroxynonan-3-one
B) 6,vii-dihydroxynonan-3-one
C) 7,8-dihydroxynonan-2-i
D) 6,7-dihydroxynonan-2-one
58 The formula of multistriatin, a pheremone of the elm bark beetle, is shown below.
What open chain ketodiol would close to this bicyclic acetal? (ignore stereoisomer bug)
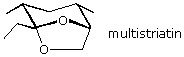
A) iii-ethyl-v-methyl-half dozen,7-dihydroxyheptan-ii-one
B) four,6-dimethyl-7,8-dihydroxyoctan-3-one
C) 4-methyl-7,8-dihydroxynonan-3-one
D) 3-ethyl-half-dozen,7-dihydroxyoctan-2-one
59 A conversion of 2,5-dimethylfuran into ii,5-dimethylpyrrole (run into equation) may be accomplished in two steps.
i) hydrolysis of the furan in aqueous acid; ii) heating the hydrolysis production with excess ammonium carbonate
What is the intermediate hydrolysis product used in the second step?
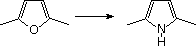
A) ii,five-hexanedione
B) 3,4-hexanedione
C) hexanedial
D) ii,five-dimethylcyclopentanone
60 The reagent semicarbazide, NH2CONHNH2, reacts with uncomplicated aldehydes and ketones (R2C=O) forming crystalline derivatives chosen semicarbazones.
Which of the following represents the construction of a semicarbazone?
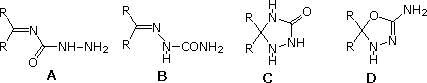 | |||
61 Which of the following compounds (I through V) should not be classified every bit an acetal?

A) II & 3
B) IV
C) I
D) none (they are all acetals)
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/questions/problems/exam8.htm
0 Response to "what starting compound(s) would you use in an aldol reaction to prepare as the major product:"
Post a Comment